Issues with nested functions
February 26, 2012 at 09:19 PM | categories: uncategorized | View Comments
Contents
Issues with nested functions
John Kitchin
Nested functions are great for passing lots of information between functions conveniently. But, there are some gotchas to be wary of!
function main
a typical nested function use
It is common to define several parameters, and then to use them inside a nested function
a = 4;
b = 3;
function y = f1(x)
y = a + b*x;
end
f1(2)
ans =
10
classic example of a potential problem
Nested functions share the same namespace as the main function, which means you can change the value of a variable in the main function in the nested function. This does not happen with subfunctions.
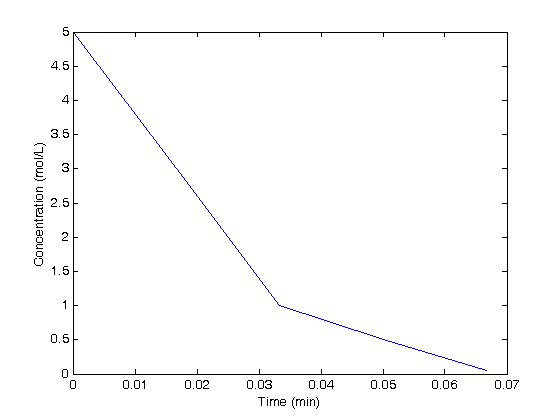
i = 5 % initial value of i function y = f2(x) % nested function that uses a and b from above, and uses an % internal loop to compute a value of y. y = 0; for i=1:10 % this is going to change the value of i outside this function y = y + a*b + i; end end f2(2) i % note i = 10 now
i =
5
ans =
175
i =
10
This kind of behavior can be very difficult to debug or even know is a problem! you have to be aware that it is an issue when using nested functions.
subfunctions
subfunctions do not share namespaces, so you can reuse variable names. This can prevent accidental variable value changes, but, you have to put the variable information into the subfunction
i = 5
f3(2)
i % note that the value of i has not been changed here.
i =
5
ans =
175
i =
5
end function y = f3(x) % subfunction that uses a and b from above, and uses an internal loop % to compute a value of y. a = 4; b = 3; y = 0; for i=1:10 % this is going to change the value of i outside this function y = y + a*b + i; end end % categories: basic
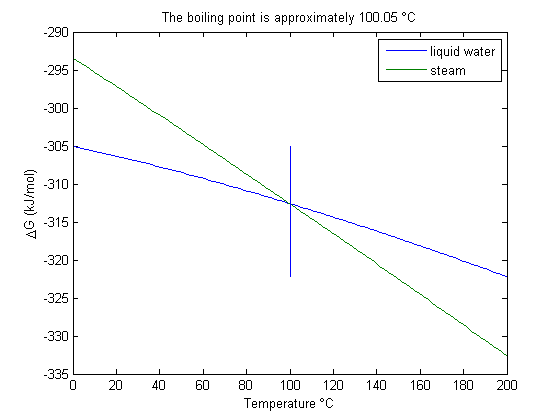
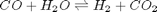 . We can illustrate the conservation of mass with the following equation:
. We can illustrate the conservation of mass with the following equation: 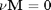 . Where
. Where  is the stoichiometric coefficient vector and
is the stoichiometric coefficient vector and 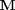 is a column vector of molecular weights. For simplicity, we use pure isotope molecular weights, and not the isotope-weighted molecular weights.
is a column vector of molecular weights. For simplicity, we use pure isotope molecular weights, and not the isotope-weighted molecular weights.