Matlab meets the steam tables
October 31, 2011 at 07:01 PM | categories: miscellaneous | View Comments
Matlab meets the steam tables
John Kitchin
A Matlab module for the steam tables is available for download at http://www.x-eng.com/XSteam_Matlab.htm. To do this example yourself, you need to download this zipfile and unzip it into your Matlab path. Today we will examine using this module for examining the Rankine power generation cycle, following example3.7-1 in Sandler's Thermodynamics book.
Problem statement: A Rankine cycle operates using steam with the condenser at 100 degC, a pressure of 3.0 MPa and temperature of 600 degC in the boiler. Assuming the compressor and turbine operate reversibly, estimate the efficiency of the cycle.
We will use the XSteam module to compute the required properties. There is only one function in XSteam, which takes a string and one or two arguments. The string specifies which subfunction to use.
We will use these functions from the module.
Contents
psat_T Saturation pressure (bar) at a specific T in degC sL_T Entropy (kJ/kg/K) of saturated liquid at T(degC) hL_T Enthalpy (kJ/kg)of saturated liquid at T(degC) vL_T Saturated liquid volume (m^3/kg) T_ps steam Temperature (degC) for a given pressure (bar) and entropy (kJ/kg/K) h_pt steam enthalpy (kJ/kg) at a given pressure (bar) and temperature (degC) s_pt steam entropy (kJ/kg/K) at a given pressure (bar) and temperature (degC)
We will use the cmu.units package to keep track of the units. Remember that the units package internally stores units in terms of base units, so to get a pressure in bar, we use the syntax P/u.bar, which is a dimensionless number with a magnitude equal to the number of bar.
clear all; clc; close all u = cmu.units;
Starting point in the Rankine cycle in condenser.
we have saturated liquid here, and we get the thermodynamic properties for the given temperature.
T1 = 100; % degC P1 = XSteam('psat_T',T1)*u.bar; s1 = XSteam('sL_T', T1)*u.kJ/u.kg/u.K; h1 = XSteam('hL_t', T1)*u.kJ/u.kg; v1 = XSteam('vL_T', T1)*u.m^3/u.kg; % we need this to compute compressor work
isentropic compression of liquid to point 2
The final pressure is given, and we need to compute the new temperatures, and enthalpy.
P2 = 3*u.MPa; s2 = s1; % this is what isentropic means T2 = XSteam('T_ps',P2/u.bar, s1/(u.kJ/u.kg/u.K)); h2 = XSteam('h_pt', P2/u.bar,T2)*u.kJ/u.kg; % work done to compress liquid. This is an approximation, since the % volume does change a little with pressure, but the overall work here % is pretty small so we neglect the volume change. WdotP = v1*(P2-P1); fprintf('the compressor work is: %s\n', WdotP.as(u.kJ/u.kg,'%1.2f'))
the compressor work is: 3.02*kJ/kg
isobaric heating to T3 in Boiler where we make steam
T3 = 600; % degC P3 = P2; % this is what isobaric means h3 = XSteam('h_pt', P3/u.bar, T3)*u.kJ/u.kg; s3 = XSteam('s_pt', P3/u.bar, T3)*u.kJ/u.kg/u.K; Qb = h3 - h2; % heat required to make the steam fprintf('the boiler heat duty is: %s\n', Qb.as(u.kJ/u.kg,'%1.2f'))
the boiler heat duty is: 3260.68*kJ/kg
isentropic expansion through turbine to point 4
Still steam
T4 = XSteam('T_ps',P1/u.bar, s3/(u.kJ/u.kg/u.K)); h4 = XSteam('h_pt', P1/u.bar, T4)*u.kJ/u.kg; s4 = s3; % isentropic Qc = h4 - h1; % work required to cool from T4 to T1 in the condenser fprintf('the condenser heat duty is: %s\n', Qc.as(u.kJ/u.kg,'%1.2f'))
the condenser heat duty is: 2316.99*kJ/kg
This heat flow is not counted against the overall efficiency. Presumably a heat exchanger can do this cooling against the cooler environment, so only heat exchange fluid pumping costs would be incurred.
to get from point 4 to point 1
WdotTurbine = h4 - h3; % work extracted from the expansion fprintf('the turbine work is: %s\n', WdotTurbine.as(u.kJ/u.kg,'%1.2f'))
the turbine work is: -946.72*kJ/kg
efficiency
This is a ratio of the work put in to make the steam, and the net work obtained from the turbine. The answer here agrees with the efficiency calculated in Sandler on page 135.
eta = -(WdotTurbine - WdotP)/Qb
fprintf('the overall efficiency is %1.0f%%\n', (eta*100))
eta = 0.291271696419079 the overall efficiency is 29%
Entropy-temperature chart
The XSteam module makes it pretty easy to generate figures of the steam tables. Here we generate an entropy-Temperature graph.
T = linspace(0,800,200); % range of temperatures figure; hold on % we need to compute S-T for a range of pressures. Here for P = [0.01 0.1 1 5 30 100 250 500 1000] % bar % XSteam is not vectorized, so here is an easy way to compute a % vector of entropies S = arrayfun(@(t) XSteam('s_PT',P,t),T); plot(S,T,'k-') text(S(end),T(end),sprintf('%1.1f bar',P),'rotation',90) end set(gca,'Position',[0.13 0.11 0.775 0.7]) % saturated vapor and liquid entropy lines svap = arrayfun(@(t) XSteam('sV_T',t),T); sliq = arrayfun(@(t) XSteam('sL_T',t),T); plot(svap,T,'r-') plot(sliq,T,'b-') xlabel('Entropy (kJ/(kg K)') ylabel('Temperature (^\circC)')
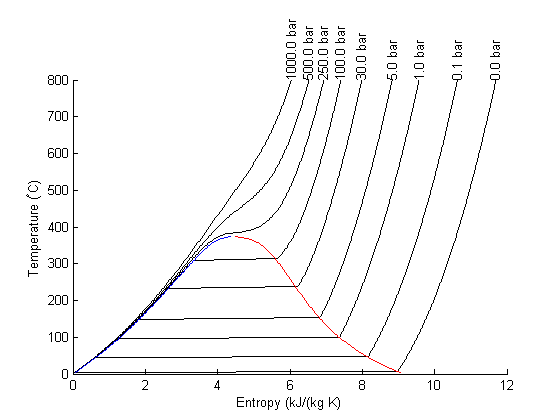
Plot Path
The path from 1 to 2 is isentropic, and has a small T change.
plot([s1 s2]/(u.kJ/u.kg/u.K),... [T1 T2],'ro ','markersize',8,'markerfacecolor','r') % the path from 2 to 3 is isobaric T23 = linspace(T2, T3); S23 = arrayfun(@(t) XSteam('s_PT',P2/u.bar,t),T23); plot(S23,T23,'b-','linewidth',4) % the path from 3 to 4 is isentropic plot([s3 s4]/(u.kJ/u.kg/u.K),... [T3 T4],'g-','linewidth',4) % and from 4 to 1 is isobaric T41 = linspace(T4,T1-0.01); % subtract 0.01 to get the liquid entropy S41 = arrayfun(@(t) XSteam('s_PT',P1/u.bar,t),T41); plot(S41,T41,'r-','linewidth',4)
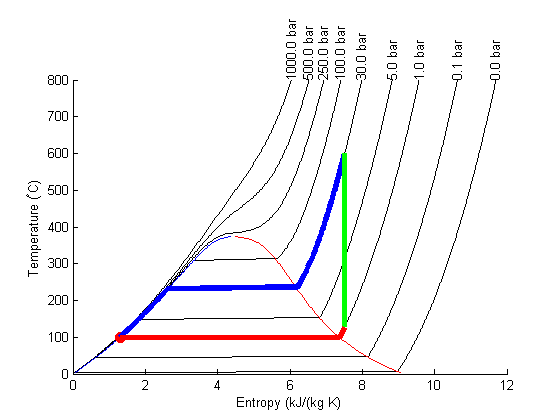
Note steps 1 and 2 are very close together on this graph and hard to see.
Summary
This was an interesting exercise. On one hand, the tedium of interpolating the steam tables is gone. On the other hand, you still have to know exactly what to ask for to get an answer that is correct. The XSteam interface is a little clunky, and takes some getting used to. It would be nice if it was vectorized to avoid the arrayfun calls.
% categories: Miscellaneous % tags: Thermodynamics